Parameters
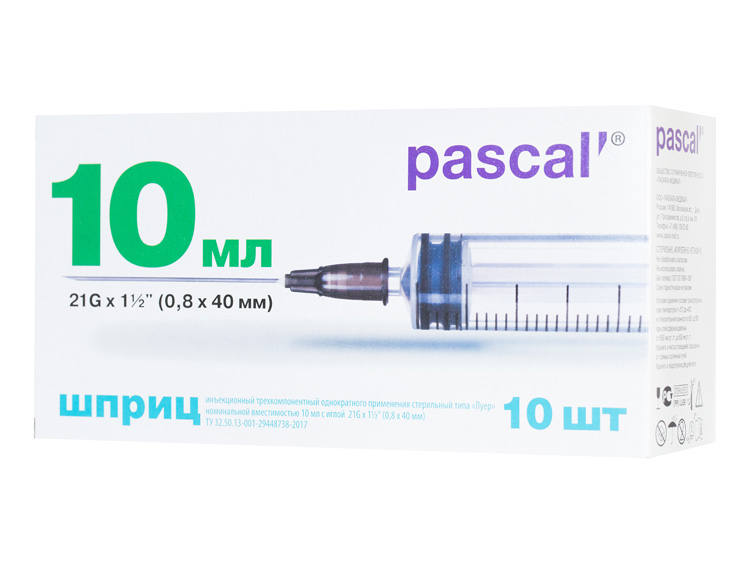
| Volume, ml: | 10 ml |
| Connection type: | Luer |
| Needle size: | 21G х 1 ½" (0.8х40 mm) |
Description
Three-component hypodermic sterile single-use syringes with a Luer slip, nominal capacity 10 ml, with a concentric tip, correspond to GOST ISO 7886-1. The syringes include a barrel with a silicone inner coating, a plunger with a gasket (seal) and a needle put on with a protective cap. The barrel material is Polypropylene. The finger flange has no burrs and sharp edges. The barrel is completely transparent for a good visualization of the contained volume. The scale with a numeration every ten marks is easy to read, accurate, located strictly parallel to the vertical axis of the barrel and is resistant to flushing and abrasion during use. Graduation interval: 0.5 ml.
The double lock ring in the internal surface of the barrel prevents occasional extraction of the plunger during drawing up medication. The plunger made of medical polypropylene has notches at its base for it possible forced destruction. The plunger stopper (seal) is made of Trans Poly-isoprene (hypoallergenic material and latex free), silicone coated for a smooth and uniform plunger movement. The seal has rings for contacting with the inner surface of the barrel that excludes leakage. The syringe is equipped with one injection needle put on with a standard cut that is triangular sharpened and silicone coated for atraumatic insertion and to reduce pain during a penetration in tissues. Needle size is 21G х 1 ½″ (0.8х40 mm).
The colour coding of the needles meets the requirements of GOST ISO 6009. The needle cannula is made of stainless steel (AISI 304). The safety cap with five enforcement ribs that guarantees a comfort opening and, respectively, add-on security to medical staff during removing a cap from the needle and reduces risk of contamination of the needle during removing a cap, also procures saving of time to fulfil a simple medical manipulation as “injection”.
The individual sterile blister package with turn-up edge (peel-pack) is made of medical grade paper and transparent film (a film composition is Polyethylene / Polyamide). The blister is opened with little effort preventing spontaneous opening, without the formation of paper fringe. Non-toxic, non-pyrogenic. The sterilization method is ethylene oxide. The sterilization period is 5 years.

-min.jpg)
-min.jpg)